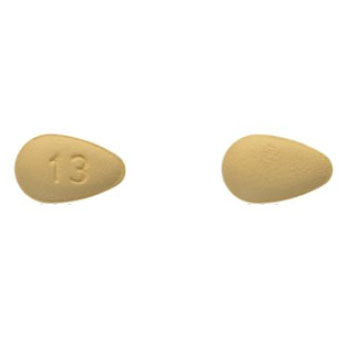
Taro RX product details
Product Information
-
DIN:02452073
-
Active Ingredients :tadalafil
-
Innovator Name:Cialis®
-
Category:cGMP-Specific Phosphodiesterase Type 5 Inhibitor
-
Dosage form:Tablet
-
Strength:5 mg
- Package size:
28 Blister - UPC Code:
876515002736 -
Non-medicinal ingredients :Anhydrous lactose, Croscarmellose sodium, Sodium lauryl sulphate, Hydroxyl propyl cellulose, Polysorbate 80, Magnesium Stearate, Hypromellose, lactose monohydrate, Titanium dioxide, Triacetin, Talc and Iron oxide Yellow. Additionally 2.5 mg tablet contains Iron oxide red and 10 mg tablet contains Iron oxide Black.
All brand names and trademarks referenced remain the property of their respective owners.
To view the available Product Monographs for Taro Pharmaceuticals Inc. and TaroPharma products please click here to access the Health Canada Drug Product Database and search by DIN or other listed criteria. You may also contact us to request the information.
For Provincial Formulary Status click here
Product Information
-
DIN:02452111
-
Active Ingredients :tadalafil
-
Innovator Name:Cialis®
-
Category:cGMP-Specific Phosphodiesterase Type 5 Inhibitor
-
Dosage form:Tablet
-
Strength:20 mg
- Package size:
4 Blister - UPC Code:
876515002750 -
Non-medicinal ingredients :Anhydrous lactose, Croscarmellose sodium, Sodium lauryl sulphate, Hydroxyl propyl cellulose, Polysorbate 80, Magnesium Stearate, Hypromellose, lactose monohydrate, Titanium dioxide, Triacetin, Talc and Iron oxide Yellow. Additionally 2.5 mg tablet contains Iron oxide red and 10 mg tablet contains Iron oxide Black.
All brand names and trademarks referenced remain the property of their respective owners.
To view the available Product Monographs for Taro Pharmaceuticals Inc. and TaroPharma products please click here to access the Health Canada Drug Product Database and search by DIN or other listed criteria. You may also contact us to request the information.
For Provincial Formulary Status click here



 Taro Corporate
Taro Corporate Taro U.S.A
Taro U.S.A Taro Canada
Taro Canada Taro Israel
Taro Israel Sun Pharma
Sun Pharma Chattem Chemicals Inc.
Chattem Chemicals Inc.